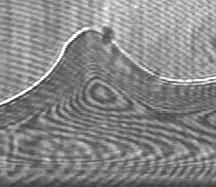

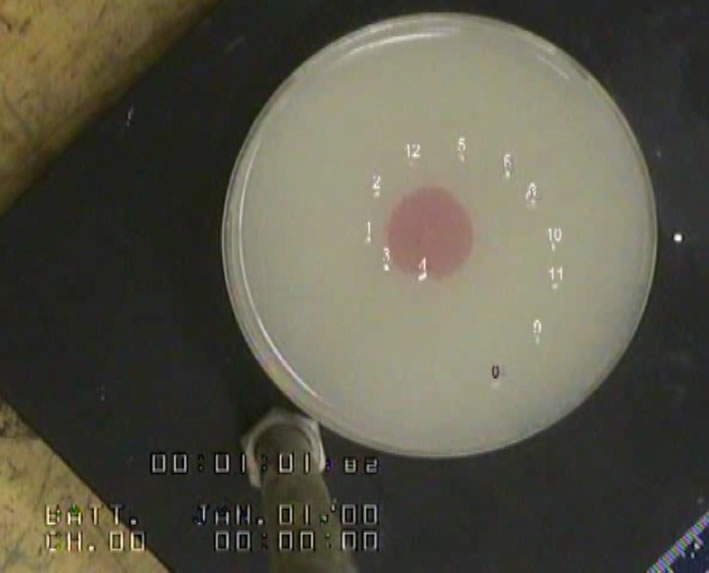
 |
The
properties of interfacial regions where fluid or solid phases meet
dominate the behavior of many natural and technical processes.
Wetting,
friction and adhesion, corrosion, stability of emulsions (droplets of
liquids in another liquid) or colloidal suspensions (solid particles
suspended in a liquid) are
some examples. But attaining a fundamental
understanding of their behavior challenges our experimental abilities
because these interfacial regions are structurally complex on a
molecular scale, are seldom homogeneous on a microscopic scale, and may
not even be in equilibrium.
Often monomolecular layers along the
interfaces dominate the behavior of the interface and the macroscopic
phenomenon.
In
the Interfacial
Physics Group we attempt to build an understanding of interfacial
phenomena on the molecular, microscopic, and macroscopic levels. In our
research, we probe many different liquid systems, including aqueous and
non-aqueous fluids and solutions, surfactants and polymers, and even
metals interacting with a variety of solids, including glasses, oxides
and metals. We employ a range of techniques including x-ray, neutron,
and optical techniques, atomic force microscopy, rheology, as well as
UHV and non-UHV materials
preparation. Presently, we focus on wetting,
friction, and colloidal
forces. Our program draws on a broad range of
scientific phenomena such as random field effects, nonequilibrium
states, hydrodynamics, and noise in hysteretic systems. The results of
our research reveal the scientific
underpinnings of such
technologies
as coatings, adhesion, colloidal stability, multiphase fluid flow and
drug delivery in the lung.
|
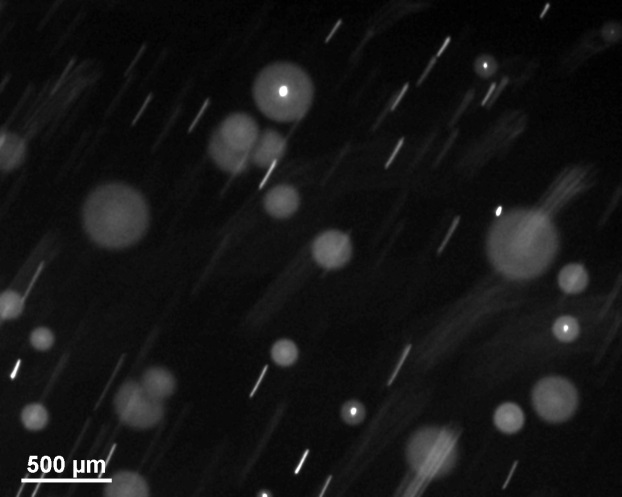

 |



