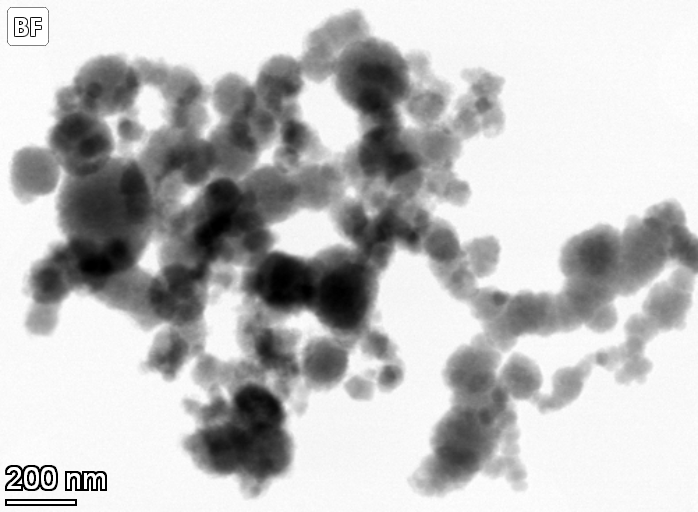
Nanoparticles for Cleaner Water
Groundwater is a vital resource for many people in their daily lives. It supplies drinking water for half of the American population, and the majority of it is used in the irrigation of agriculture. But groundwater is susceptible to contamination, whether from industry, agriculture, gasoline, or general chemicals such as road salt. One method for groundwater remediation is to inject materials underground to degrade those contaminants.
For more than two decades, the material nanoscale zero valent iron (NZVI) has been used for groundwater remediation. It is a strong reductant that dechlorinates industrial solvents and reduces heavy metals in groundwater. However, it does not discriminate, also reacting with the water itself and reducing it to form hydrogen gas that fizzes away. Researchers throughout the field searched for a way to alter the material so it would react with water less and the contaminants more.
In recent years, sulfidized nanoscale zerovalent iron (SNZVI) has become a promising reactive material for this process. Researchers found that if sulfur was added to NZVI—to form SNZVI—the problem was solved: the particles became much less reactive with water and more reactive with target contaminants. However, little was previously understood about how the added sulfur altered the nanoscale zerovalent iron and how it interacted with the groundwater. This prevented the rational design of robust SNZVI for specific applications.
“What was missing was the ability to understand how the sulfur incorporated into the particles and how the form of sulfur in those particles affected their performance,” said Greg Lowry, professor of civil and environmental engineering. In March of 2020 Lowry published a paper in Advanced Materials exploring the reactivity and selectivity of SNZVI during the remediation of trichloroethylene (TCE), a hydrophobic groundwater contaminant.
Lowry and his colleagues conducted experiments by incorporating different amounts of sulfur into NZVI particles. They analyzed the resulting SNZVI particles’ properties, including hydrophobicity, electron transfer, and sulfur speciation, and how SNZVI interacted with TCE. “This paper has, for the first time, understood systematically the sulfur content and speciation in the particles,” said Lowry.
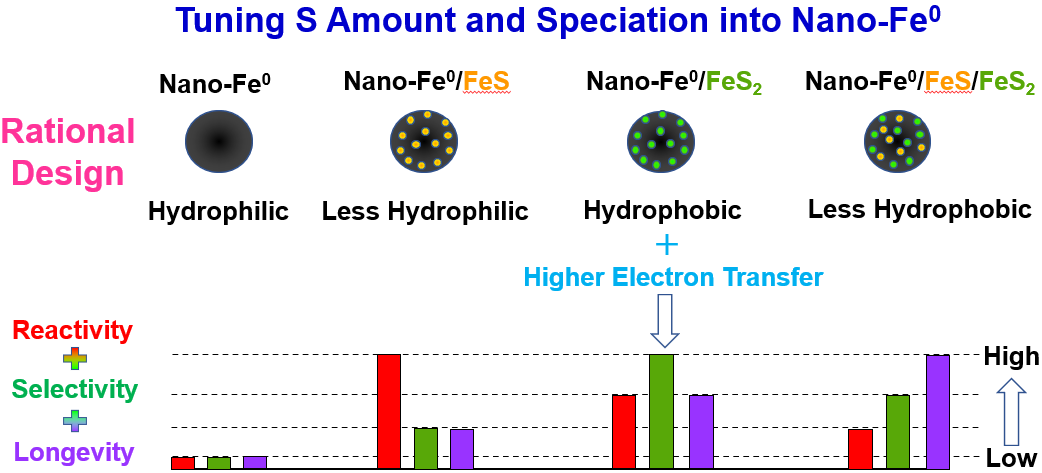
The results of the experiments reveal that sulfur on the SNZVI surfaces makes them hydrophobic—less favorable to react with water. It also prevents hydrogen absorption, which is a necessary step in forming hydrogen gas, and improves electron transfer to the chlorinated solvent. This combination of factors makes the material less reactive with water and more reactive with trichloroethylene.
The benefits of using sulfur in materials for groundwater remediation are now clear. Additionally, while some other types of materials could garner similar results, sulfur is non-toxic, natural, and cost-effective. With Lowry and his team’s findings, people can now use SZNVI for specific applications; they can synthesize or make a sulfidized material with the properties tailored to their groundwater, reaction, and contaminant conditions.
“This offers a new class of nano-reactive material for groundwater remediation that will be much more effective than the previous class of materials using just NZVI,” said Lowry. “I think the next chapter in the entire groundwater nano-iron industry is going to be this sulfidized nano-iron.”
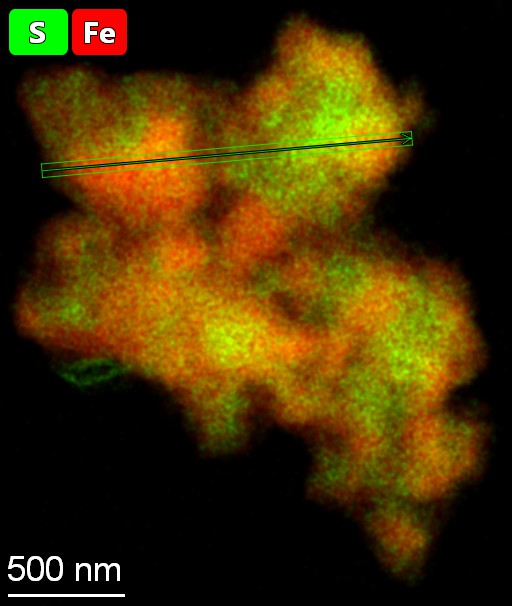 Lowry collaborated with experts from several academic institutions across multiple disciplines, including density functional theory and x-ray photoelectron spectroscopy, to conduct research for this paper.
Lowry collaborated with experts from several academic institutions across multiple disciplines, including density functional theory and x-ray photoelectron spectroscopy, to conduct research for this paper.
“This is a good representation of a collaborative effort, where multiple domains of expertise were applied to a problem, a mix of experimental work and materials design and modeling,” he said. “This allowed us to not just make a material, but to understand how it worked.”