Biologist Receives Curci Foundation Grant To Investigate New Strategy for Treating Pain
Fundamental Research Could Lead to Creation of Non-Addictive Alternatives to Opioids
By Jocelyn Duffy / 412-268-9982 / jhduffy@andrew.cmu.edu
Manojkumar Puthenveedu is an associate professor of biological sciences at CMU.
Carnegie Mellon University Associate Professor of Biological Sciences Manojkumar Puthenveedu has received a $200,000 grant from the Shurl & Kay Curci Foundation to support his research into the cellular mechanisms that underlie pain and addiction to painkillers. The project could be a critical step toward a new, non-addictive way to treat pain.
Chronic pain affects hundreds of millions of people in the United States and over a billion people around the world. Pain management drugs represent a large portion of the market, and the costs exceed that of heart disease, cancer, and diabetes combined. Opioids like morphine, which target the mu opioid receptor on the surface of neurons, are the main drugs prescribed to treat pain. These drugs, however, have severe limitations. They are not very effective in treating chronic pain, and their use often leads to tolerance and addiction — a serious and rapidly growing problem in the current world. According to the Centers for Disease Control and Prevention (CDC), prescriptions and sales of opioids have quadrupled since 1999 and more than 40 Americans die each day from prescription opioid overdoses — a 100 percent increase in the last 10 years. This epidemic in opioid abuse led the CDC to issue new, stricter opioid prescribing guidelines for primary care providers when treating patients for chronic pain.
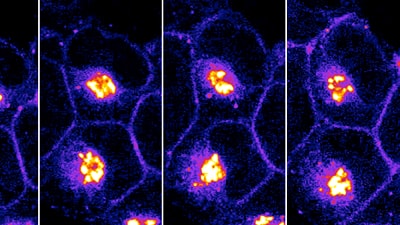
Transport of a modified DOR sensor to the cell surface.
Under the new grant, Puthenveedu will investigate an alternate drug target to the mu opioid receptor. His target is the delta opioid receptor (DOR), which also can inhibit pain pathways when on the surface of neurons. DOR is a highly attractive target because the drugs that activate DOR are not addictive. However, many drugs that have been developed to target DOR proved to be ineffective for managing pain in a clinical setting. Puthenveedu noted that the bulk of DOR appears to collect in storage pools inside neurons, keeping it from moving to the surface. If DOR is not on the surface, a DOR-targeting drug can't bind to it, and therefore will not be effective.
To counter this problem, the Puthenveedu lab has begun to identify the mechanisms that retain DOR inside the cell. In their proof-of-concept experiments, they found a way to mobilize the storage pool and drive DOR to the cell surface. This approach significantly improves the effectiveness and utility of DOR-targeting molecules, and could prove to be transformative in treating pain.
"To develop this novel approach of engineered relocation followed by activation, it is imperative that we fully understand the mechanisms mediating DOR storage and delivery in neurons. With the support of the Curci Foundation, we can begin to do that," said Puthenveedu, who is a member of the joint Carnegie Mellon and University of Pittsburgh Center for the Neural Basis of Cognition and Carnegie Mellon's BrainHub neuroscience initiative.
Puthenveedu anticipates that his research on DOR will be relevant to other clinically important receptors. DOR belongs to the G protein-coupled receptor (GPCR) family. GPCRs are the targets for the majority of drugs available to treat a variety of ailments, especially neuropsychiatric disorders. Puthenveedu plans to use his research on DOR as a jumping off point to investigate the cellular storage and delivery mechanisms of other GPCRs — a largely unexplored area.
The Curci Foundation also will continue to fund Carnegie Mellon research that is teaching mice to use two-dimensional brain-computer interfaces. Led by Aryn Gittis and Sandra Kuhlman, assistant professors of biological sciences, and Assistant Professor of Biomedical Engineering Steven Chase, the project could provide a way for scientists to link neural plasticity and behavioral improvement, and will provide a model for research into the neural basis of memory, learning, behavior and motor control. The model also could be used to develop new methods of rehabilitation for brains injured by stroke, traumatic brain injury or diseases like Parkinson's disease.
As the birthplace of artificial intelligence and cognitive psychology, Carnegie Mellon has been a leader in the study of brain and behavior for more than 50 years. The university has created some of the first cognitive tutors, helped to develop the Jeopardy-winning Watson, founded a groundbreaking doctoral program in neural computation, and completed cutting-edge work in understanding the genetics of autism. Building on its strengths in biology, computer science, psychology, statistics and engineering, CMU launched BrainHub, a global initiative that focuses on how the structure and activity of the brain give rise to complex behaviors.